Endovascular surgery highlights
Regarding the mobile hybrid OR and a deep dive into vascular case planning
Navigating the change - Customer Insights
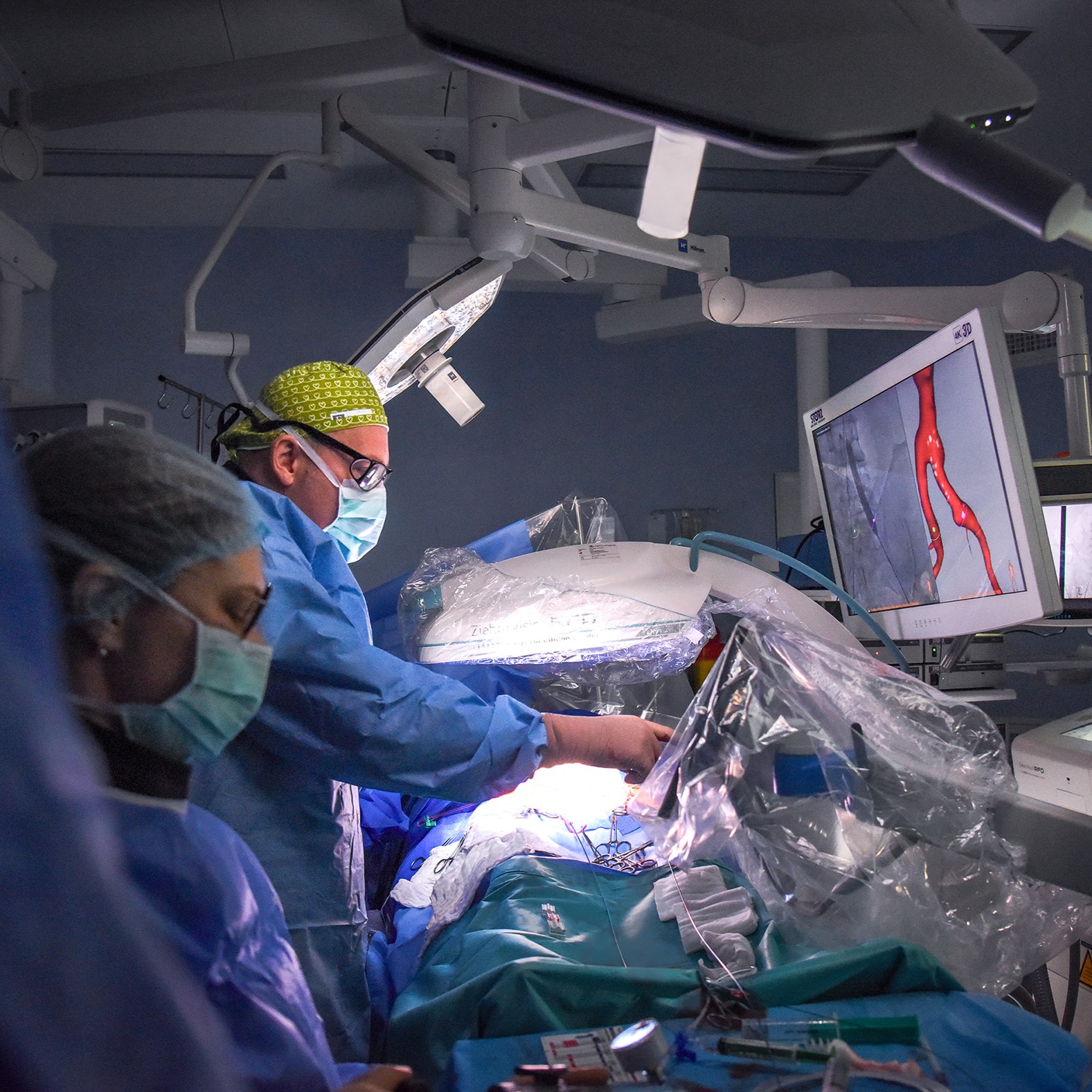
Read how the team at the Sanador Clinic, led by Professor Dr. Victor Costache, offers cost-effective and patient-friendly healthcare solutions that successfully address the ongoing societal change.
Panorama - Clinical Report
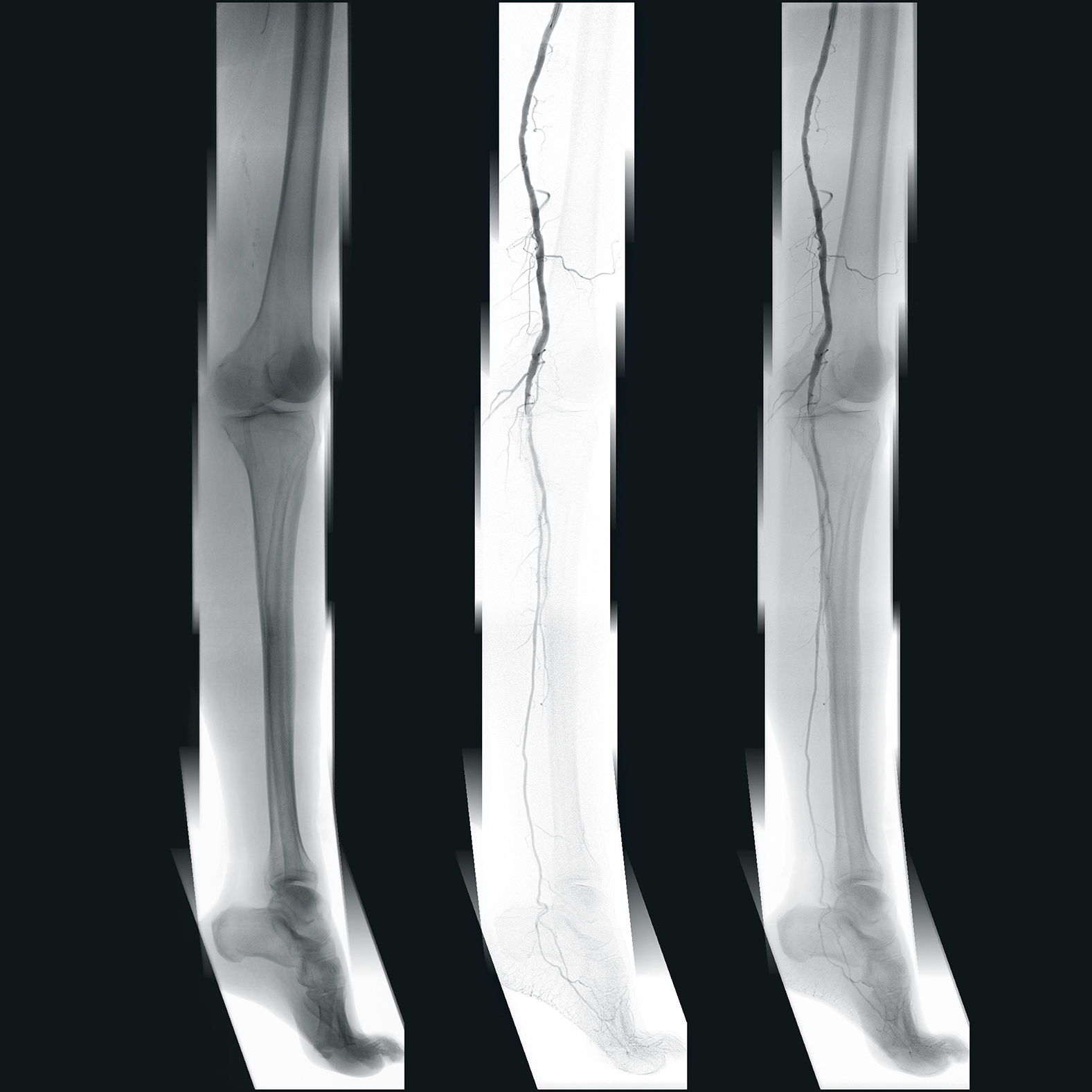
Discover how the intraoperative navigation system EndoNaut1 supports the team of vascular surgeons at Giessen University Hospital, Germany, during their procedures and provides them with a constant overview in 2D panoramic images of the extremities.

Case planning in minutes - Tips and Tricks
Get to know proven methods for the EndoSize2 software solution and learn how it enables accelerated, AI-based preparation of the procedure on the computer – to streamline vascular surgery with reduced use of radiation and contrast agents.
Dichiarazione di non responsabilità
1 EndoNaut® is a registered trademark of Therenva SAS. In the USA, the EndoNaut® software obtained a substantial equivalence determination and FDA clearance through the CDRH premarket notification process (510(K)). In Europe, the EndoNaut® software is CE marked (class IIb), not eligible for reimbursement. The information provided in the labelling and manual is intended for healthcare professionals only. For the safe and successful operation and use of the device, always read the instructions.
2 EndoSize® is a registered trademark of Therenva SAS. In the USA, the EndoSize® software obtained a substantial equivalence determination and FDA clearance through the CDRH premarket notification process (510(K)). In Europe, the EndoSize® software is CE marked (class IIa), not eligible for reimbursement. The information provided in the labelling and manual is intended for healthcare professionals only. For the safe and successful operation and use of the device, always read the instructions.