EndoSize
The intuitive software by Therenva for planning cases in minutes
The intuitive software by Therenva for planning cases in minutes
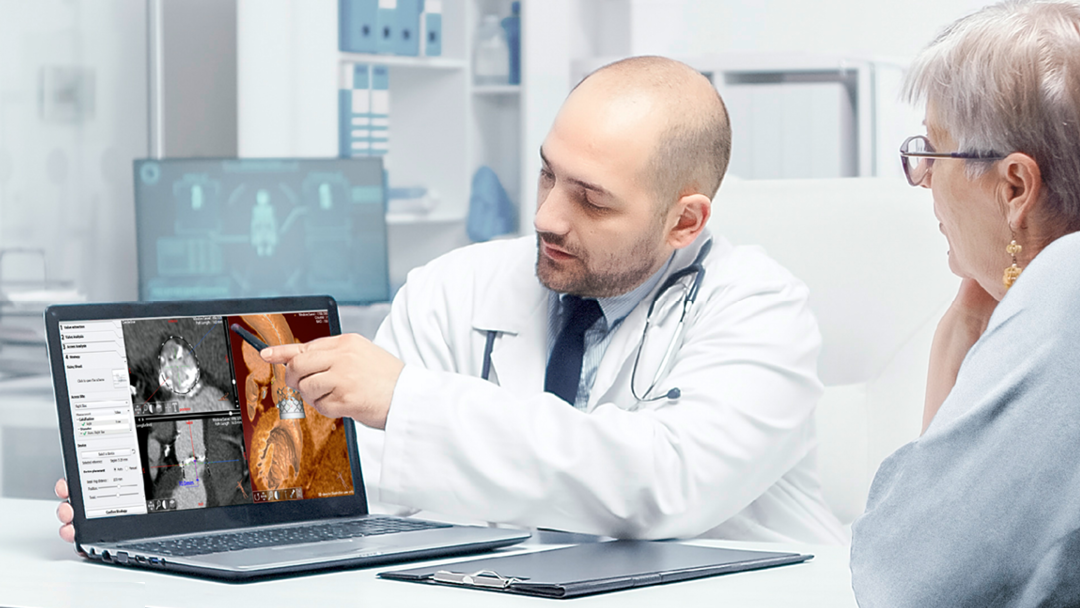
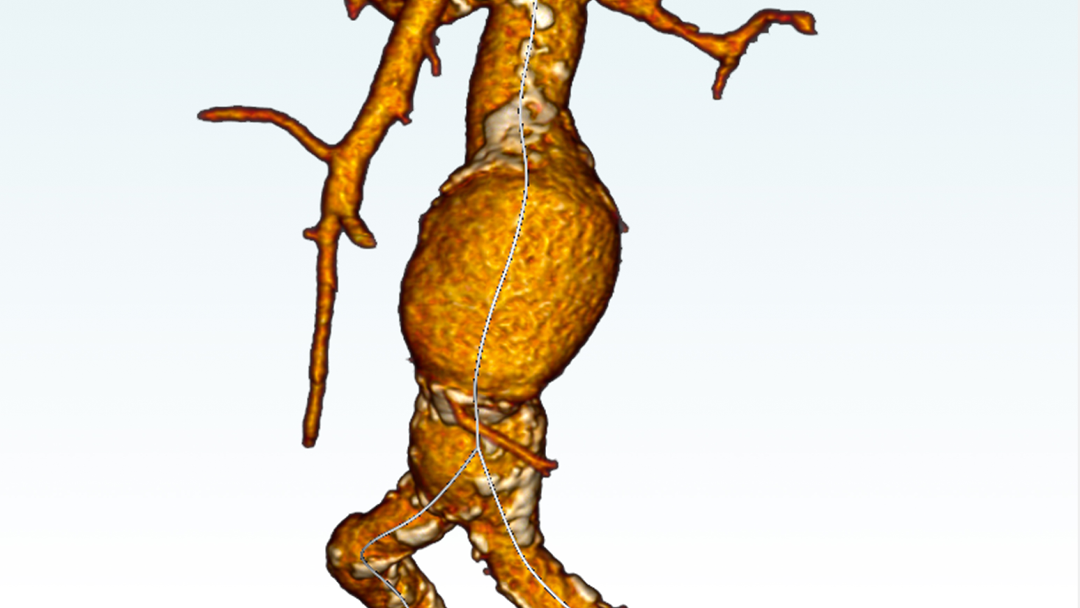
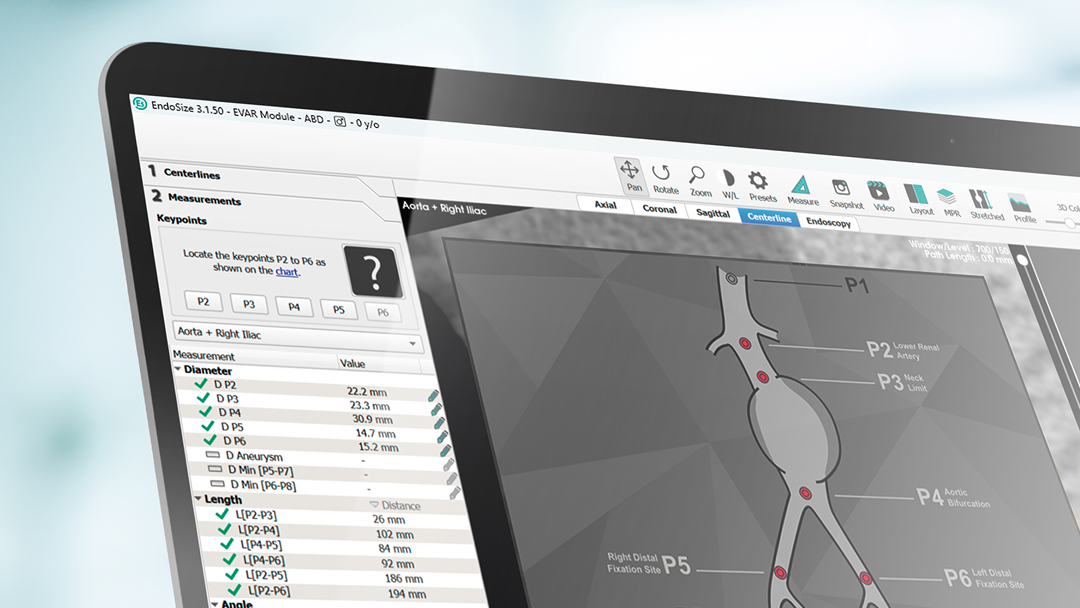
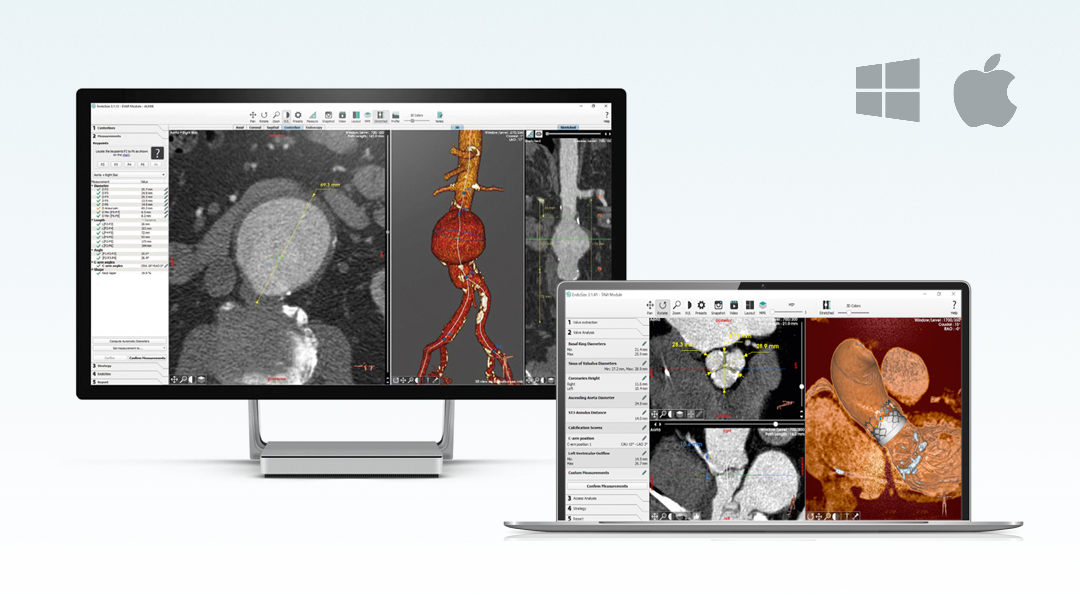
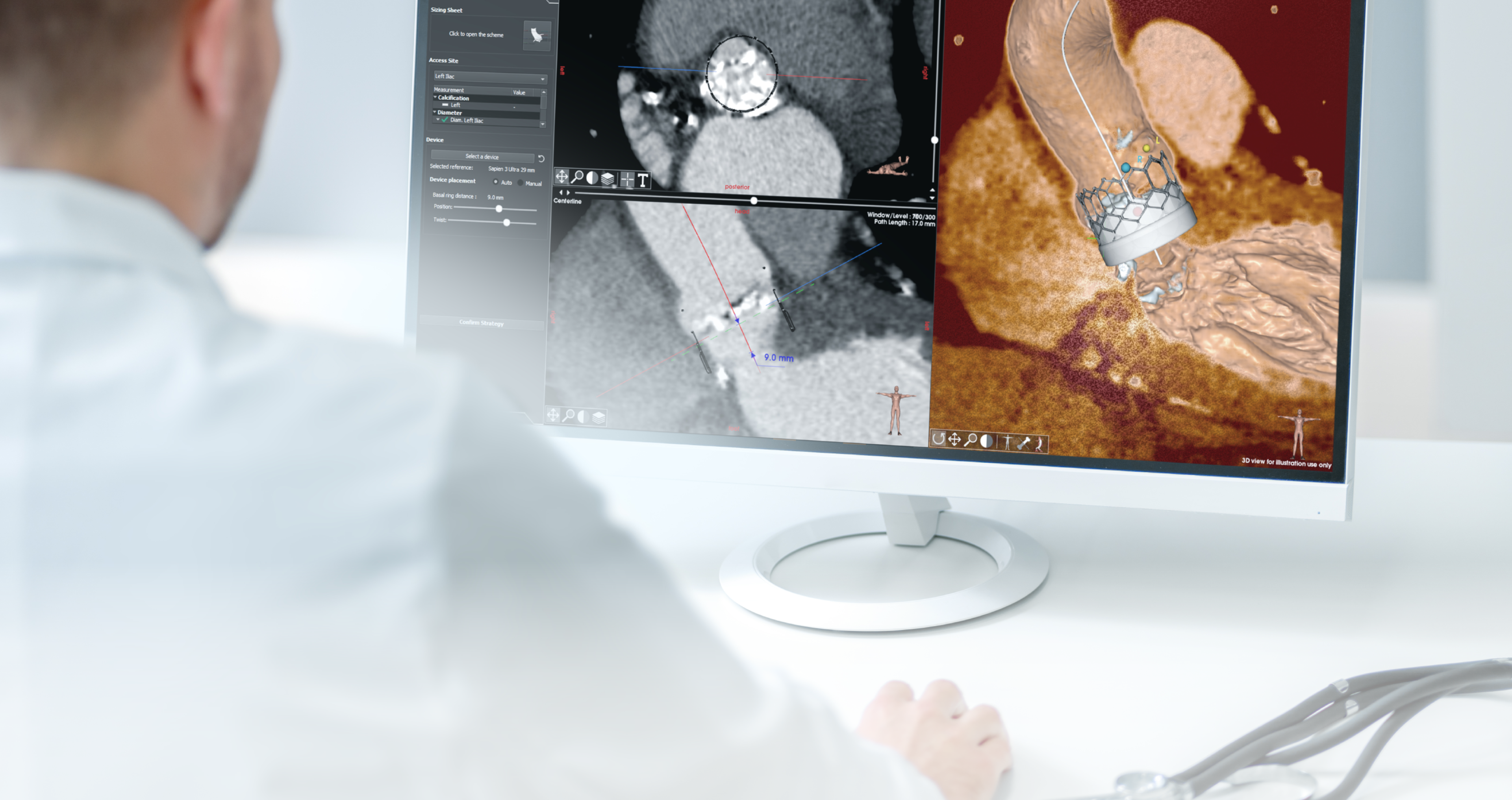
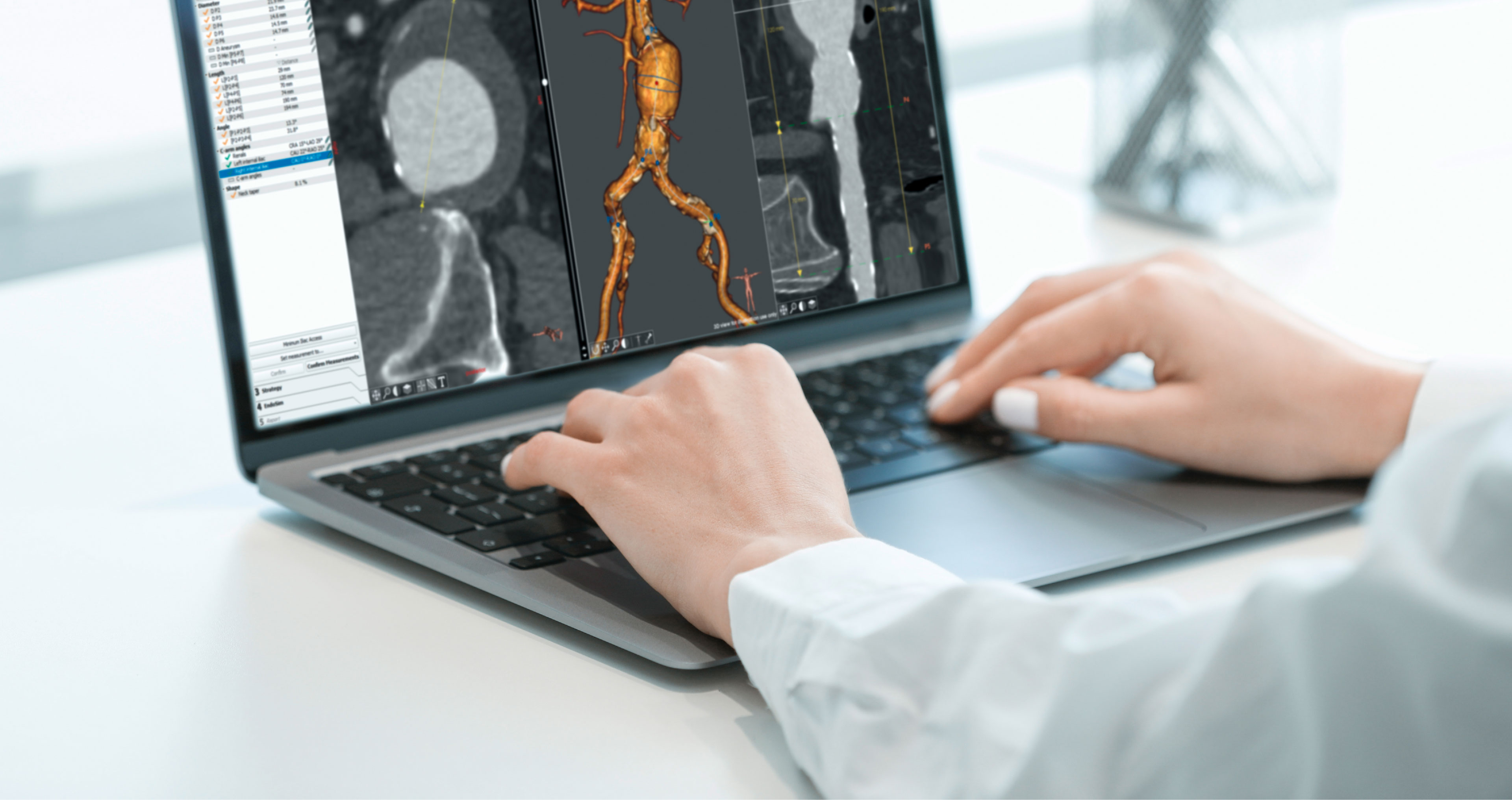
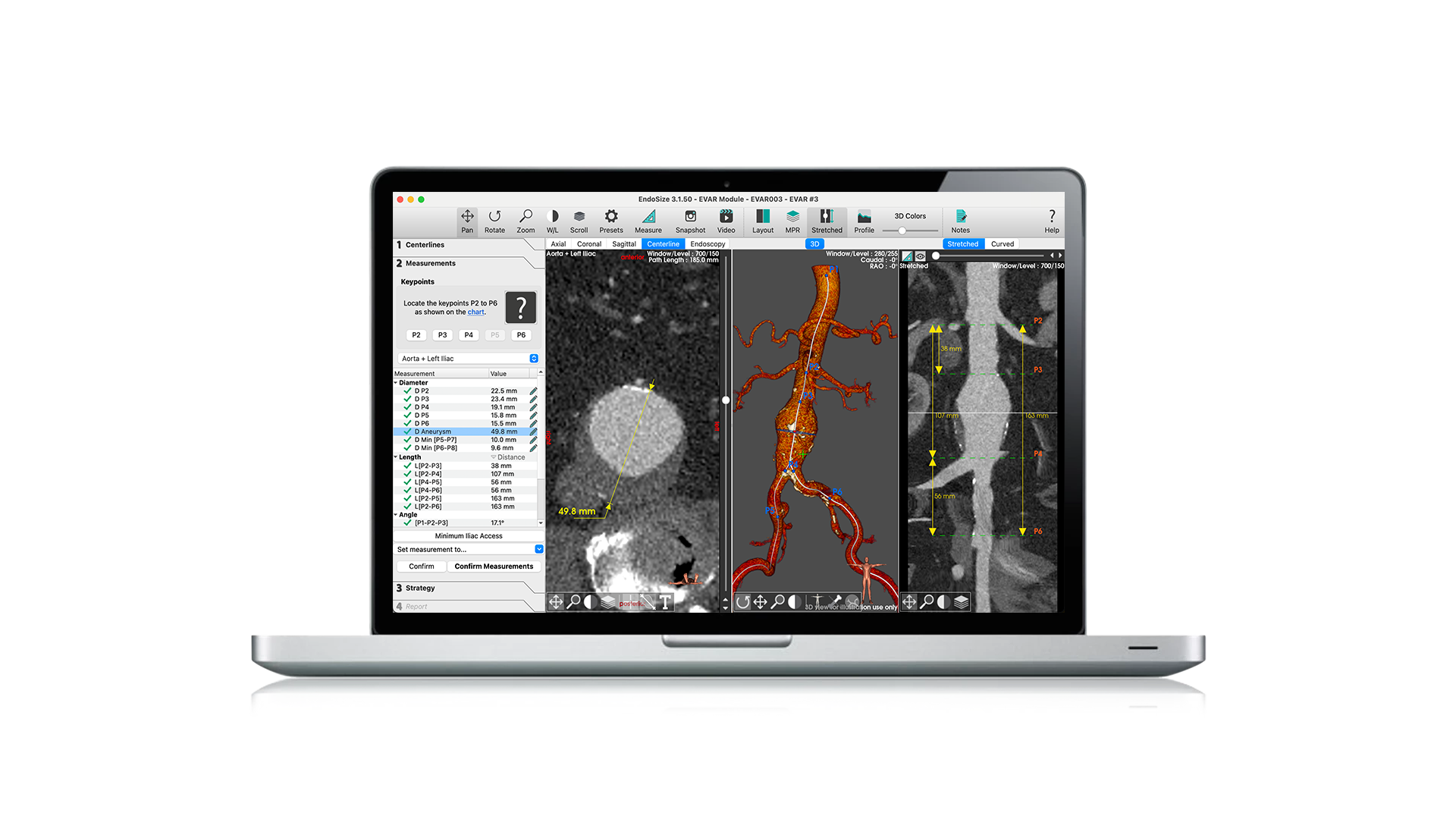
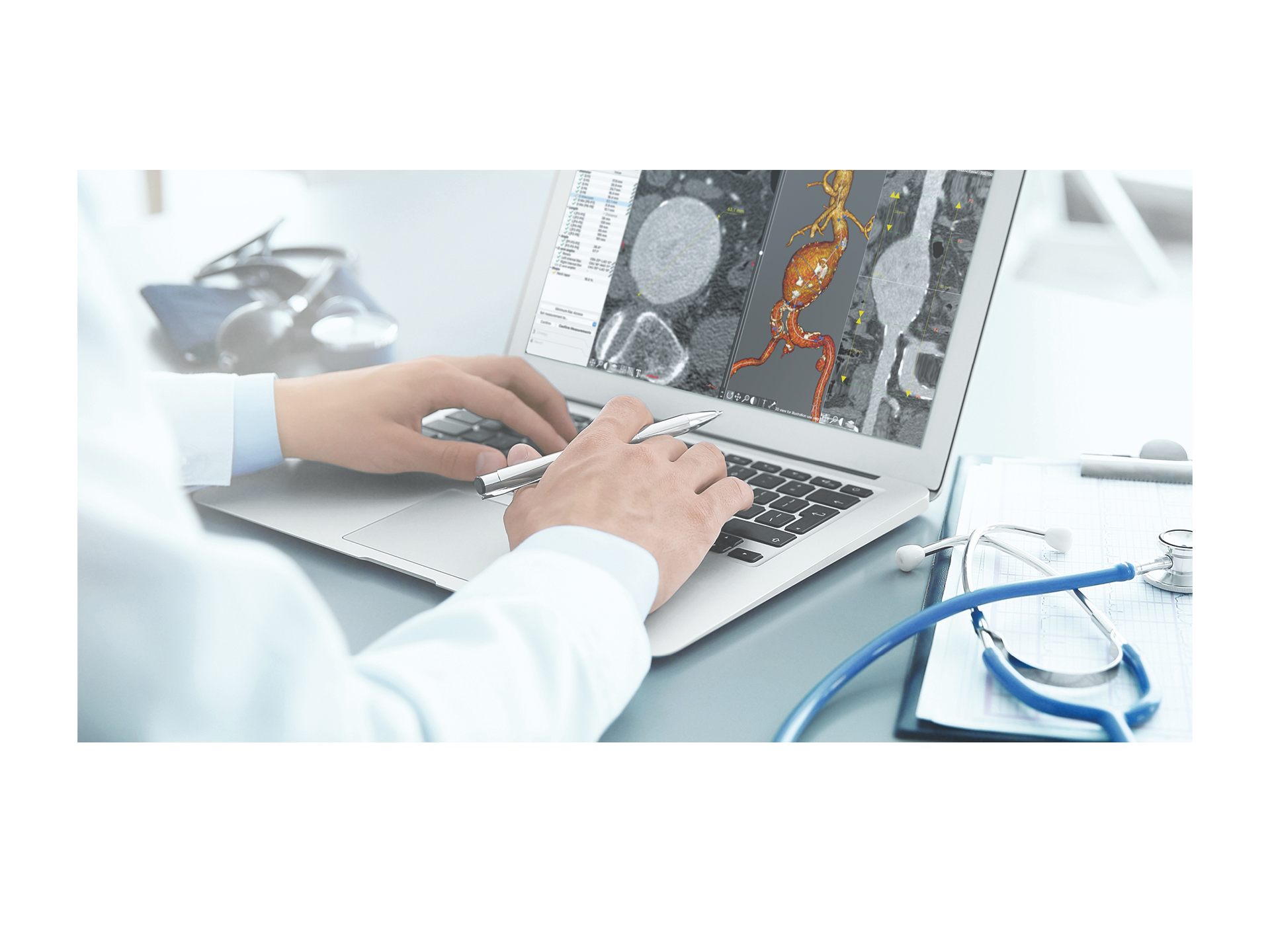
EndoSize1 is a dedicated case planning software that allows surgeons to fully plan the treatment strategies and placement of devices. It accurately measures the anatomy based on CT images, suggests the appropriate endograft designs, and defines all other relevant parameters. Cases can be planned within a few minutes and clinicians can easily select the most suitable device from a comprehensive catalog, and view all the technical specifications and order information.
Interested in learning more?
We are looking forward to getting your inquiry!
Disclaimer
-
1
EndoSize® is a registered trademark of Therenva SAS. In the USA, the EndoSize® software obtained a substantial equivalence determination and FDA clearance through the CDRH premarket notification process (510(K)). In Europe, the EndoSize® software is CE marked (class IIa), not eligible for reimbursement. The information provided in the labelling and manual is intended for healthcare professional