EndoNaut
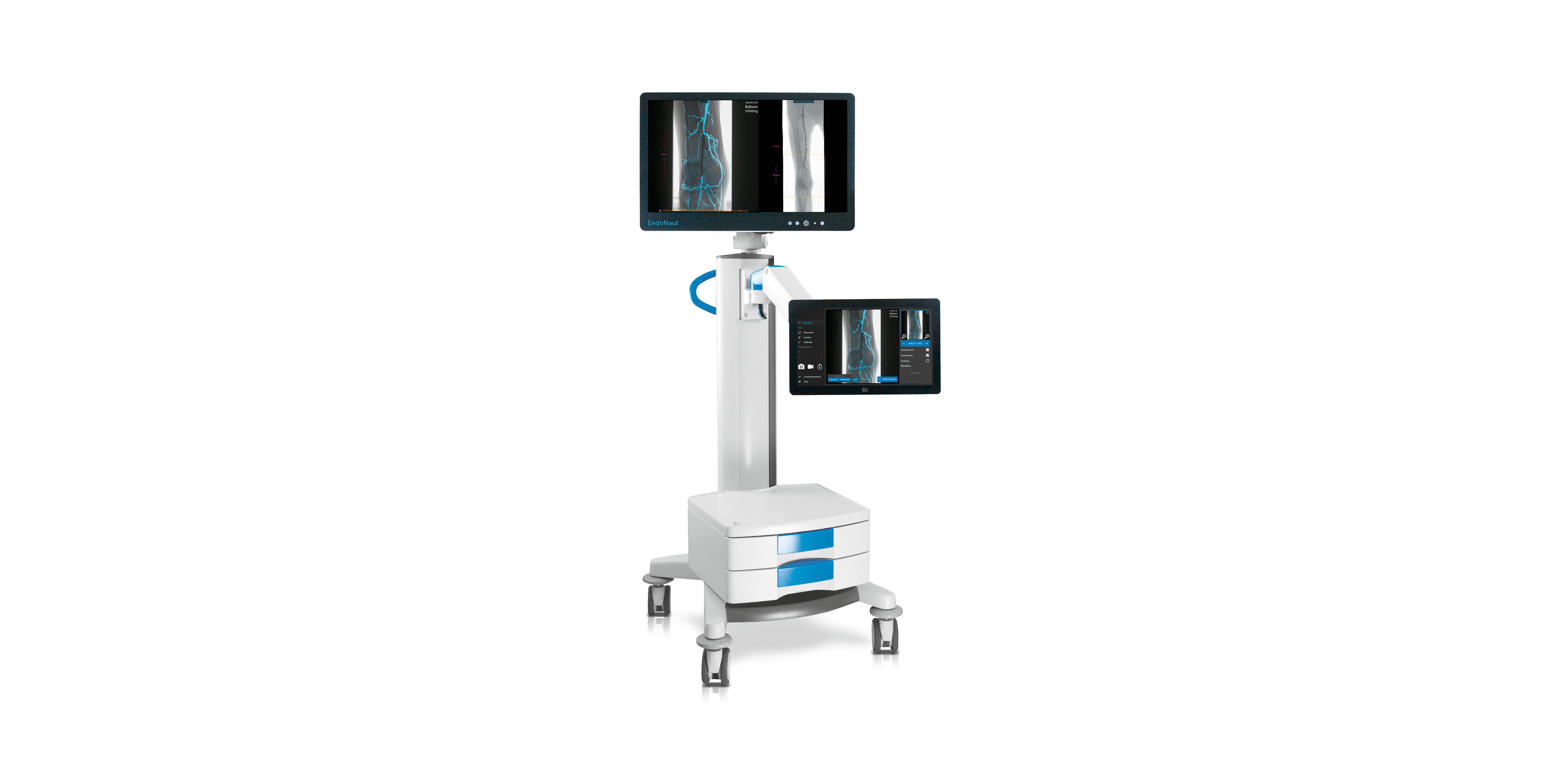
The intraoperative navigation system with image fusion for optimized endovascular procedures
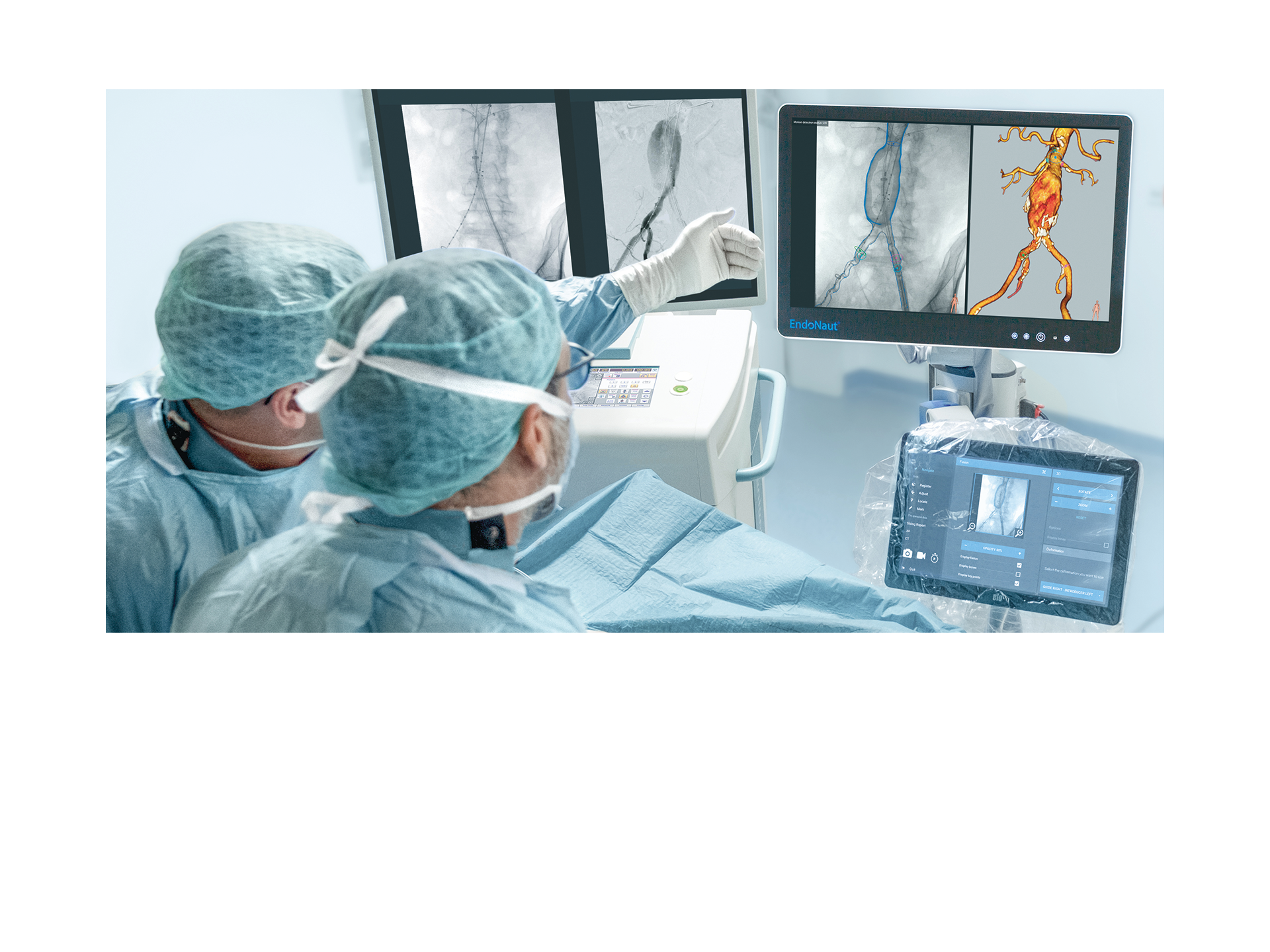
The intraoperative navigation system with image fusion for optimized endovascular procedures
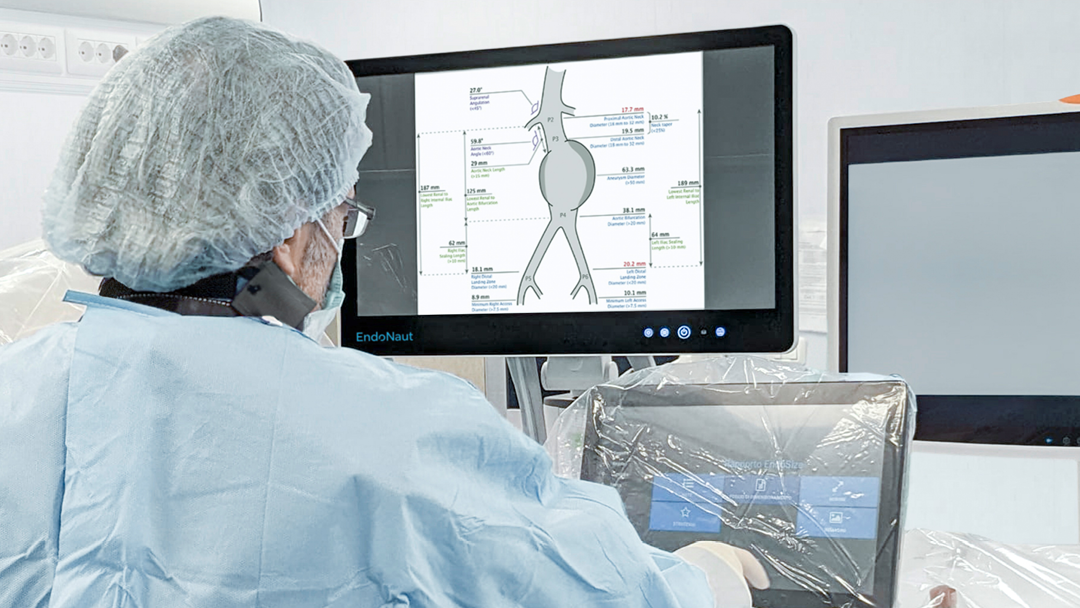
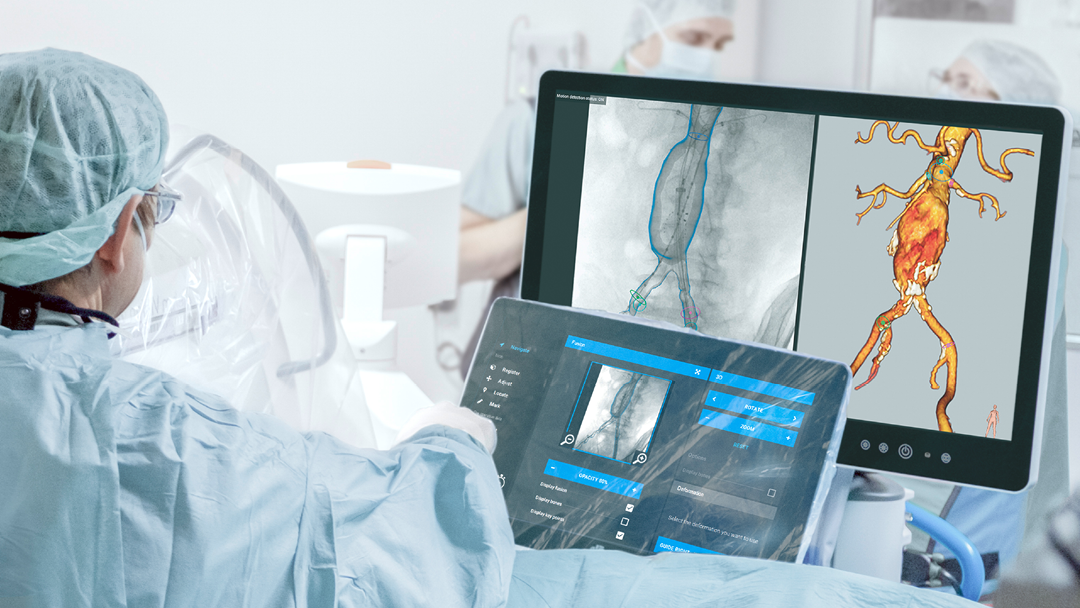
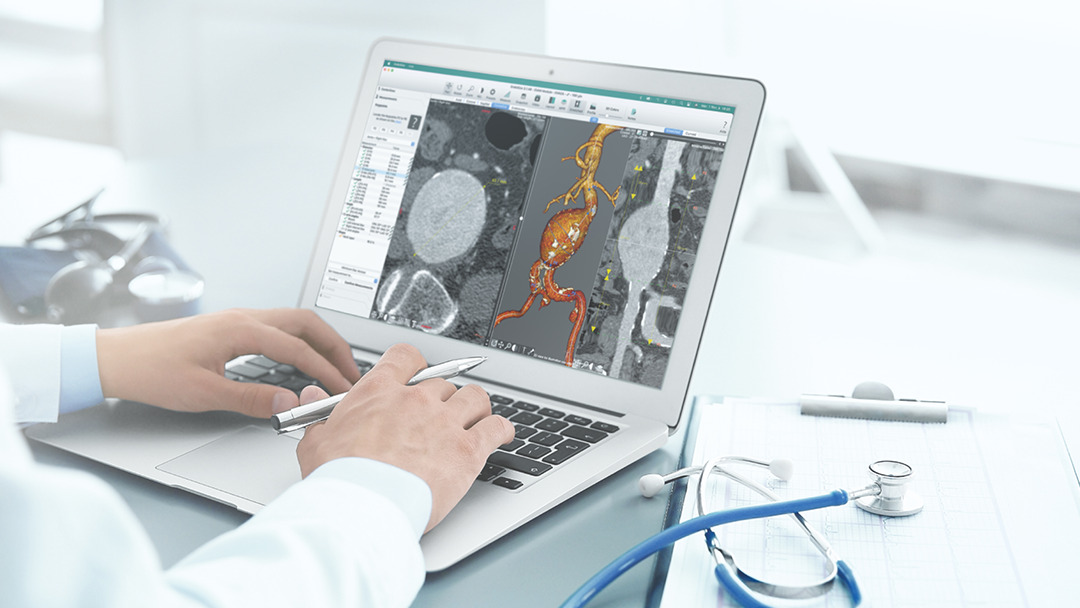
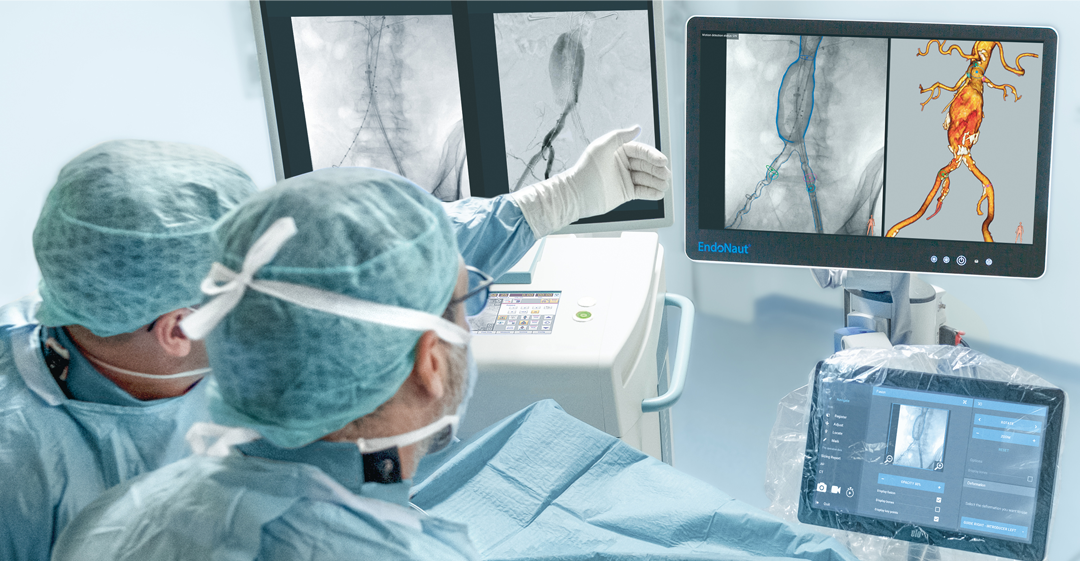
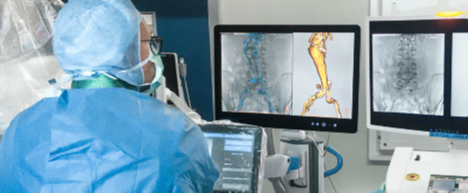
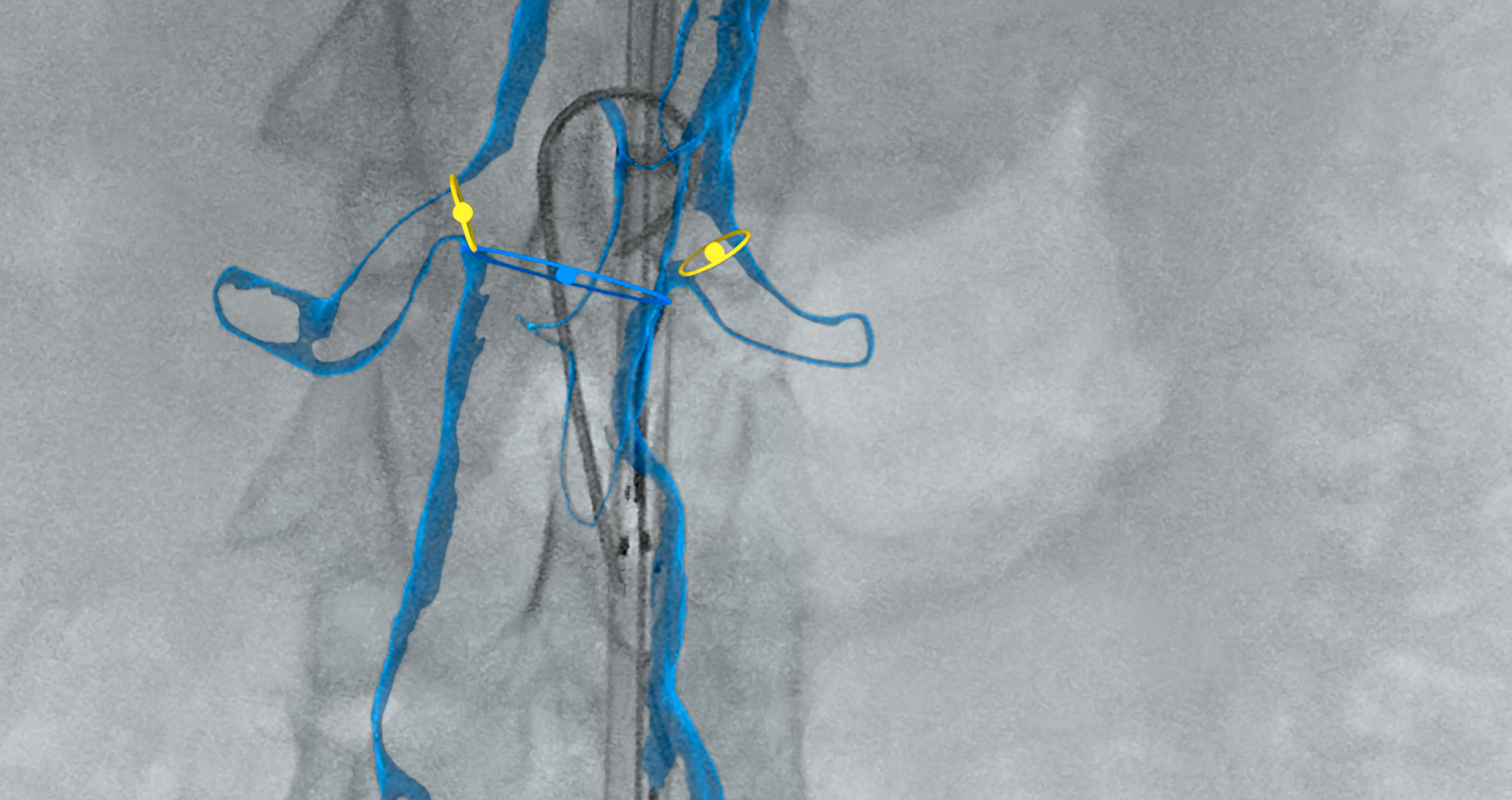
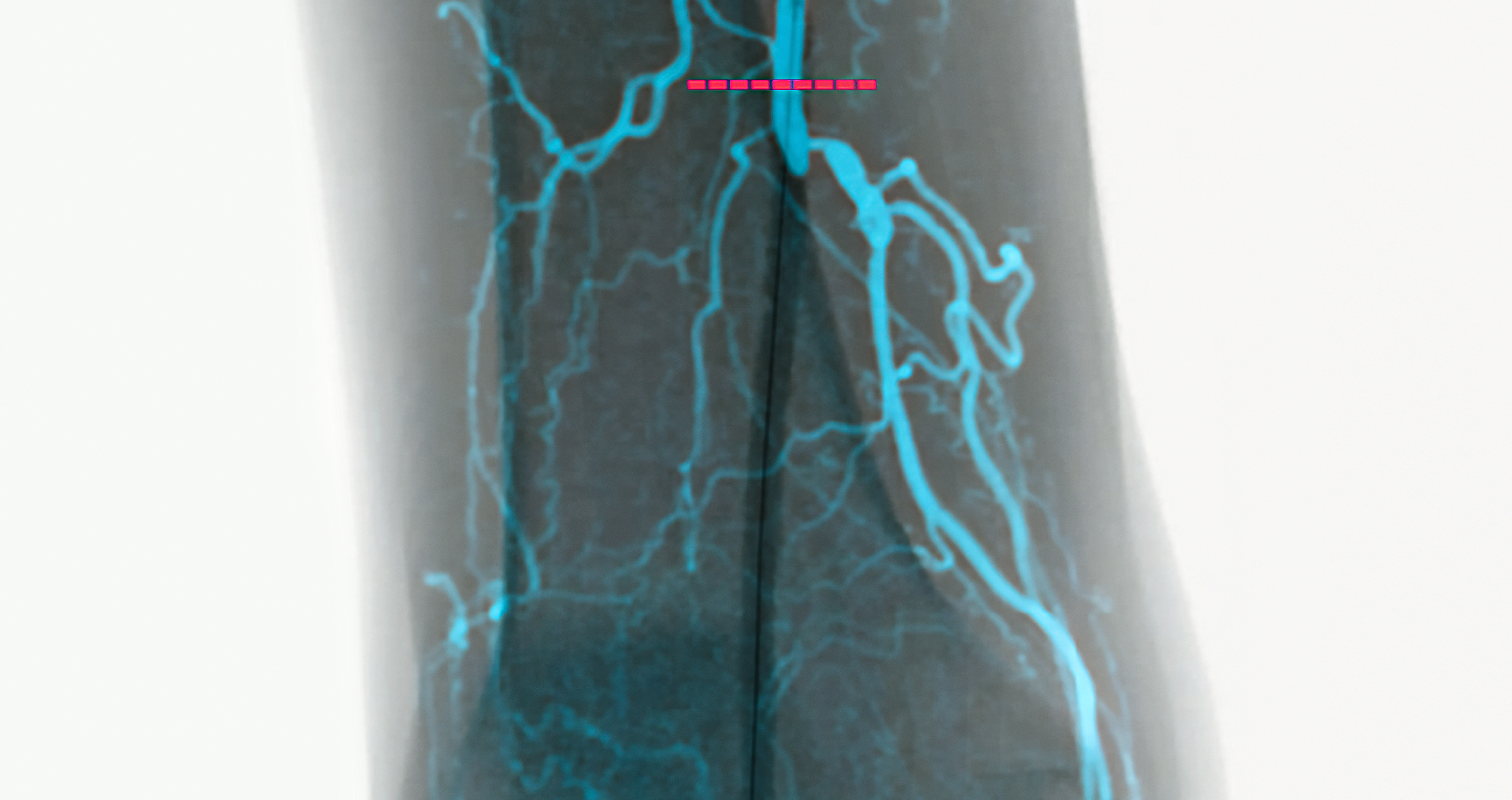
EndoNaut1 is an intraoperative navigation system that supports surgeons during endovascular procedures. Using image fusion technology, it enables precise visualization for accurate interventions, reducing the X-ray dose and the use of contrast media. It is compatible with every C-arm in the OR, whether permanently installed/mobile or digital/analog.
Two different modules are available: one for aortoiliac procedures (AI) and one specifically for procedures on the lower extremities (PAD).
Interested in learning more?
We are looking forward to getting your inquiry!
Disclaimer
-
1
EndoNaut® is a registered trademark of Therenva SAS. In the USA, the EndoNaut® software obtained a substantial equivalence determination and FDA clearance through the CDRH premarket notification process (510(K)). In Europe, the EndoNaut® software is CE marked (class IIb), not eligible for reimbursement. The information provided in the labelling and manual is intended for healthcare professionals only. For the safe and successful operation and use of the device, always read the instructions.